What to Expect from New Chronic Weight Management Medications


by Craig Primack, MD, FACP, FAAP
Fall 2013
Please Note: As of February 2020, the Food and Drug Administration has issued a voluntary withdrawal of Belviq, Belviq XR (lorcaserin) from the U.S. market, as a safety clinical trial has shown an increased occurrence of cancer. Patients currently taking Belviq have been advised to stop taking the medication and speak with their healthcare provider about its continued use.
In 2012, two new medications were approved by the U.S. Food and Drug Administration (FDA) for chronic weight management. These drugs have been approved for both men and women with a body mass index (BMI) greater than 30 or greater than 27 with one or more medical conditions, such as high blood pressure, high blood lipids, diabetes, heart disease or sleep apnea. Both medications have unique mechanisms of action and have been studied to be used with a reduced-calorie diet and regular physical activity.
Belviq®
The first medication to be approved was BELVIQ (lorcaserin HCI), manufactured by Arena Pharmaceuticals and distributed by Eisai, Inc. BELVIQ is a drug that decreases the intake of food by working on the brain’s hunger centers. It is taken two times per day with a standard dose of 10mg. Side effects of BELVIQ may include headache, dizziness, fatigue, nausea, and constipation. BELVIQ should NOT be used if you are pregnant, nursing or trying to become pregnant.
Prior to FDA approval, BELVIQ was examined in published medical studies on weight-loss. The main study showed that patients receiving 10mg twice daily for one year were more than twice as likely to lose 5 percent of their body weight as patients on placebo1. Taking BELVIQ also led to almost double the weight-loss compared to the placebo group. A separate study showed that taking BELVIQ twice a day led to greater weight-loss (~5 percent more) than taking it once per day 2.
Qsymia®
Qsymia is the second newly-approved weight-loss medication. It is a combination drug of phentermine and extended-release (ER) topiramate and is manufactured by VIVUS, Inc. This drug works in two ways. The phentermine part of the medication works on the brain to decrease hunger, and the topiramate part, commonly used in seizure control and migraine headache prevention, works to decrease the food cravings many people experience when trying to lose weight, which can be especially useful in the hours after dinner.
Qsymia has four possible dosages taken in the morning with or without food:
The starting dose (3.75mg phentermine/23mg ER topiramate) is taken for two weeks.
- After two weeks, the starting dose is increased to the recommended dose of 7.5mg phentermine/46mg ER topiramate.
- If a weight-loss of at least 3 percent is not achieved by 12 weeks of taking Qsymia, the dose may be increased again to the titration dose (11.25mg phentermine/69mg ER topiramate) for two weeks and then continuing on the top dose of 15mg phentermine/92mg ER topiramate.
- If 5 percent weight-loss is not achieved after 12 additional weeks, it is recommended to stop taking the medication.
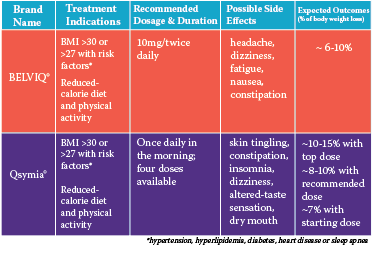 Possible side effects of Qsymia include: skin tingling, constipation, sleeplessness, dizziness, changes in the way food tastes and dry mouth. These side effects are most common when first starting the medication. ER Topiramate, a component of Qsymia, has been associated with an increased risk of birth defects if a mother is on the medication while pregnant. With this increased risk, the FDA has recommended a pregnancy test prior to starting Qsymia and continuing to test monthly while on it. This test can be done at home or in a doctor’s office. Also, two methods of birth control are recommended unless the patient has an intrauterine device (IUD), progestin implant, tubal sterilization or the male partner has a vasectomy.
Possible side effects of Qsymia include: skin tingling, constipation, sleeplessness, dizziness, changes in the way food tastes and dry mouth. These side effects are most common when first starting the medication. ER Topiramate, a component of Qsymia, has been associated with an increased risk of birth defects if a mother is on the medication while pregnant. With this increased risk, the FDA has recommended a pregnancy test prior to starting Qsymia and continuing to test monthly while on it. This test can be done at home or in a doctor’s office. Also, two methods of birth control are recommended unless the patient has an intrauterine device (IUD), progestin implant, tubal sterilization or the male partner has a vasectomy.
Qsymia has also had a number of published medical studies conducted on its use. These studies tested how well the different doses worked. Weight-loss with the top dose of Qsymia was between 6.7 and 14.7 percent after 56 weeks of the study, compared to 2.1 percent in the non-drug group3. A second study found that after 56 weeks, patients on Qsymia lost between 7.8 and 9.8 percent of body weight, depending on dose. This study was extended to 108 weeks and average weight-loss remained 9.3 to 10.5 percent compared to the non-drug group at 1.8 percent.
Conclusion
Overall, weight-loss is improved in many individuals affected by overweight or obesity when these new medications are taken in conjunction with a diet and exercise program. It is important to note that using a weight-loss medication is just one component of many tools for the chronic treatment of obesity, and they may not work for everyone. Weight-loss requires individualized treatment.
As Qsymia and BELVIQ are both new drugs, their best results will be seen throughout time as part of a comprehensive weight-loss and maintenance program that includes diet, physical activity, behavior change and adequate sleep. When considering medications for weight management, please discuss your options with your primary care physician or seek the advice of an obesity medicine specialist. You may find a specialist in your area by visiting www.FindObesityTreatment.org, which provides a free search by state, city, name or zip code.
About the Author:
Craig Primack, MD, FACP, FAAP, is co-director and co-founder of the Scottsdale Weight Loss Center, PLLC, in Scottsdale, Ariz. He is a diplomate of the American Board of Obesity Medicine and is board-certified in both internal medicine and pediatrics. Dr. Primack received his medical degree from Loyola University Stritch School of Medicine (Chicago) and completed a combined residency in internal medicine and pediatrics at Good Samaritan Regional Medical Center and Phoenix Children’s Hospital in Arizona. In 2012, Dr. Primack received the Dr. Vernon B. Astler Award from the American Society of Bariatric Physicians (ASBP) in recognition of his efforts to advance the Society’s place and purpose within the media, government and medical community. In addition, he has been the ASBP American Board of Obesity Medicine Review Course director since 2011.
References:
- Smith SR, Weissman NJ, Anderson CM, et al. Multicenter, placebo-controlled trial of lorcaserin for weight management. N Engl J Med. 2010;363:245–256.
- Fidler MC, Sanchez M, Raether B, et al. A one-year randomized trial of lorcaserin for weight loss in obese and overweight adults: The BLOSSOM trial. J Clin Endocinol Metab. 2011;96:3067–3077.
- Allison DB, Gadde KM, Garvey WT, et al. Controlled-release phentermine/topiramate in severely obese adults: a randomized controlled trial (EQUIP). Obesity (Silver Spring). 2012;20(2):330–342.
- Gadde KM, Allison DB, Ryan DH, et al. Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): a randomised, placebo-controlled, phase 3 trial. Lancet. 2011;377(9774):1341–1352. Epub 2011 Apr 8.
- Garvey WT, Ryan DH, Look M, et al. Two-year sustained weight loss and metabolic benefits with controlled-release phentermine/topiramate in obese and overweight adults (SEQUEL): a randomized, placebo-controlled, phase 3 extension study. Am J Clin Nutr. 2012;95(2):297–308. Epub 2011 Dec 7.
by Steph Wagner, MS, RDN Spring 2024 Obesity is a complex disease that often comes with other…
Read Articleby Matthew Lester Brengman, MD, MSHA, FACS Spring 2024 Exciting advancements are happening in weight management and…
Read Articleby Kendall Griffey, OAC Communications Manager Spring 2024 We have officially kicked off Your Weight Matters Regional…
Read Article









